Intrinsic Semiconductor
Definition:
An intrinsic semiconductor is a pure form of semiconductor material (like silicon or germanium) with no impurities. Its electrical conductivity comes only from the breaking of covalent bonds due to heat, which generates equal numbers of free electrons and holes.
Explanation with GIF:
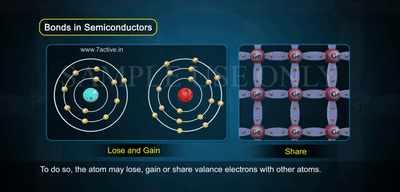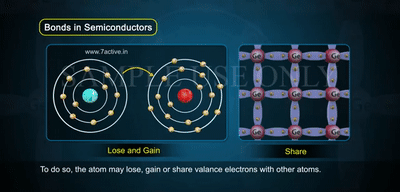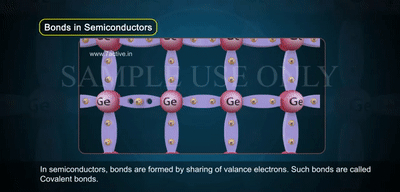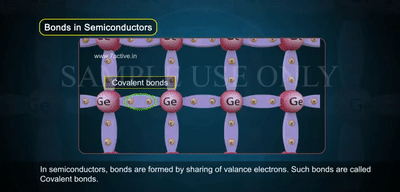
In the image, you see atoms arranged in a crystal lattice, each sharing electrons through covalent bonds.
- At low temperatures, all bonds are complete, so there are no free carriers.
- As temperature increases, some bonds break — this releases free electrons while leaving behind holes.
- Notice how electrons lose or gain energy and move, showing the balance of electrons and holes in intrinsic material.
Extrinsic Semiconductor

Definition:
An extrinsic semiconductor is formed when small amounts of impurities (dopants) are added to a pure semiconductor to increase conductivity. This process is called doping. Depending on the dopant, it becomes either n-type (electrons dominate) or p-type (holes dominate).
Explanation with image:
In the second image, you see the regular semiconductor lattice but with a different atom inserted (like Boron for p-type).
- The Boron atom has only three outer electrons, so one bond is incomplete — creating a hole.
- These holes become the majority carriers, making it a p-type semiconductor.
- If a 5-electron atom (like Phosphorus) were added, you would see an extra electron available, forming an n-type semiconductor.
⚡ Quick Recap for Users:
- Intrinsic: Pure semiconductor, equal electrons and holes.
- Extrinsic: Doped semiconductor, either p-type (holes) or n-type (electrons), much more conductive.


