What subatomic particles are found in the nucleus?- Do you know the answer? Most people will answer like proton, neutron, electron. But, is it just that? Well, it is not. This article will make you dispel the ignorance about what subatomic particles are found in the nucleus.
Molecules consist of atoms. These atoms make up normal matter. Electrons whirl around the nucleus inside atoms. Protons and neutrons make up the nucleus in most cases. However, they are composite objects. The quarks, like the electrons, are found inside protons and neutrons, but they appear to be indivisible.
History of Indivisible Particles to Divisible Particles
The physical study of subatomic particles was only conceivable throughout the twentieth century, with the advent of more modern instruments to examine the matter at scales of 10^-15 meters and smaller (that is, at distances comparable to the diameter of the proton or neutron).
However, the underlying theory of what we now know as particle physics dates back to at least 500 BCE.
- Greek philosopher Leucippus and his student Democritus were the first to tell that everything is made up of invisibly tiny, indivisible particles called atoms.
- For more than 2,000 years, the concept of atoms was widely in the dark.
- In the end, thanks to the theory that matter is made up of four elements—earth, fire, air, and water—it was then when atoms first got noticed.
People who read this article also read: True Story of How Einstein’s Brain Was Stolen for Science
How Atom Became Divisible
However, by the early nineteenth century, the atomic theory of matter was able to reappear by the work of John Dalton, an English chemist. His research was able to reveal that each chemical element consists of its own distinct type of atom. As a result, Dalton’s atoms continue to be the atoms of modern physics. By the end of the century, however, evidence began to emerge that atoms are not indivisible, according to Leucippus and Democritus. In the end, there was an establishment that atoms were rather a composition of smaller particles.
The basic structure of the atom first came into the limelight in 1911.
- Scientist Rutherford was able to demonstrate that the majority of an atom’s mass stays in its center, in a small nucleus.
- Rutherford was able to deduce that the atom was like a miniature solar system, with light, negatively charged electrons orbiting the dense, positively charged nucleus in the same way as planets circle the Sun.
- In 1913, the Danish theorist Niels Bohr modified this model by adding new quantization theories pioneered by the German physicist Max Planck at the turn of the century.
Composition of Nucleus:
- The nucleus consists of protons and neutrons. These make up the core of a particle.We can refer to them as nucleons.
- The protons have a net positive charge, the molecule’s core is positive in charge overall, and the electrons, negative in charge, rotate around the concentrated center. Inside, there is no charge on the neutrons.
- As the protons in the small core are so near to one another, the electrostatic forces of aversion also act inside the core.
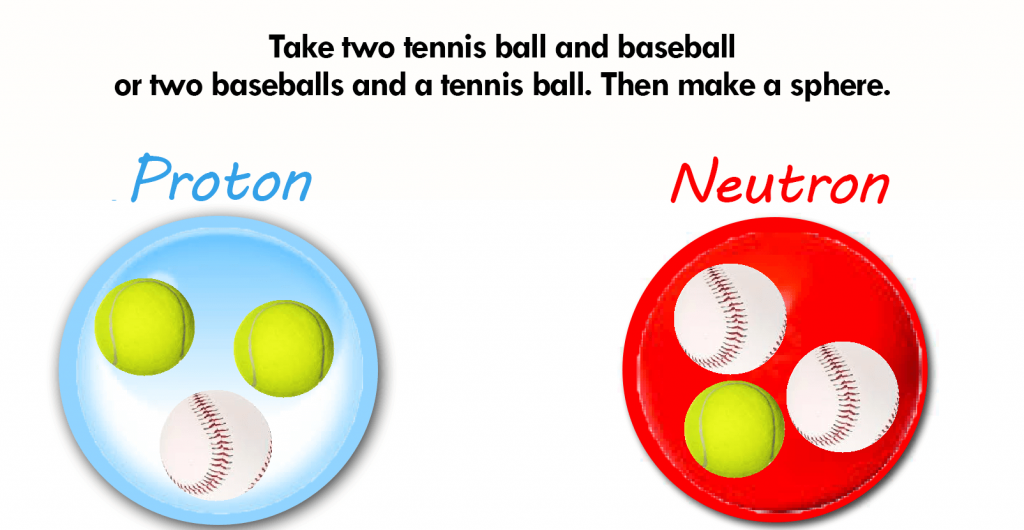
What are these Quarks?
The nucleus of an atom comes with little ball bearings known as protons and neutrons.
It turns out to be a soup of quarks in specific proportions, arranged in groups of three. There are two kinds of quarks, UP and DOWN, which correspond to the exact proportions of protons and neutrons.
The quarks account for around 2% of the nucleus’s mass, with the remainder being kinetic energy.

“What we research as the matter is not what most people think of as matter,” – Niels Bohr
The types of Quarks
As we know, atomic nuclei consist of protons and neutrons. These are both made up of three quarks (2 up quarks and 1 down quark in a proton, 1 up quark, and 2 down quarks in a neutron). These are the matter particles (fermions) that contribute to the formation of a nucleus. There are also gluons, which are force particles (bosons). It serves to keep the quarks in the protons and neutrons together in the nucleus. Another form of boson is the “weak force” or “intermediate vector” boson. It has involvement in nuclear decay (W and Z bosons).
People who read this article also read: How Long is Infinity? What are 10^100 Times of Infinity?
The 4 Basic Particles:
On a nuclear or global scale, quarks and leptons are the building blocks of matter, but they require some type of mortar to bind themselves together into more complex cases.
- The particles that make up this mortar stay in link to four fundamental forces. These forces are what we together know as matter’s fundamental interactions.
- Gravity (or gravitational force), the electromagnetic force, and two forces that scientists are better familiar with: the strong force and the weak force are the four basic forces.
- The two forces, strong and weak nuclear forces, have only subatomic, if not subnuclear, effects.
- Quarks in protons, neutrons, and other subatomic particles stay together by the strong force.
- Similarly to how the electromagnetic force holds bulk stuff together, the strong force holds protons and neutrons together within atomic nuclei.
- The weak force operates on both quarks and leptons, unlike the strong force, which only acts between quarks.
- The nuclear events that power the Sun and other stars are driven by this force, which is responsible for the beta decay of a neutron into a proton.
Conclusion
I hope you enjoyed my ultimate guide to your question: what subatomic particles are found in the nucleus.
Now I’d like to hear from you: what do you know about subatomic particles? Did you know the 4 fundamental particles? Do you think Quarks have divisions too?
Either way, let me know by leaving a quick comment below.